(CUSTOM) Peptid Sentezi

Peptide production Service
A peptide consists of a compound, either natural or synthetic, that contains two or more amino acids linked by a carboxyl group of one of the amino acids and the amino group of another. Peptide molecules are structurally the same as proteins,but are generally smaller. There are many peptides that function in diverse ways, for example as hormones, antibiotics and other compounds which participate in many of the metabolic activities of living organisms.
The chemical synthesis of peptides has been used for many years as an effective tool for both research and diagnostic purposes. In the past, liquid phase synthesis, or “Solution Phase Peptide Synthesis” was performed. However, advances in the knowledge of the chemical basis of the reaction have led to a synthesis of peptides using a solid phase, “Solid Phase Peptide Synthesis”.
– Solid Phase Peptide Synthesis. This peptide synthesis method is based on the incorporation of N-α-amino acids into the sequence of any required peptide anchored to a solid matrixwhich acts as a support. In the peptide synthesis process, soluble reagents are generally washed awayat the end of the addition (of each amino acid). Once the desired peptide is obtained, it is freed from the polymeric support.
The general scheme for peptide synthesis is as follows (Figure 1):
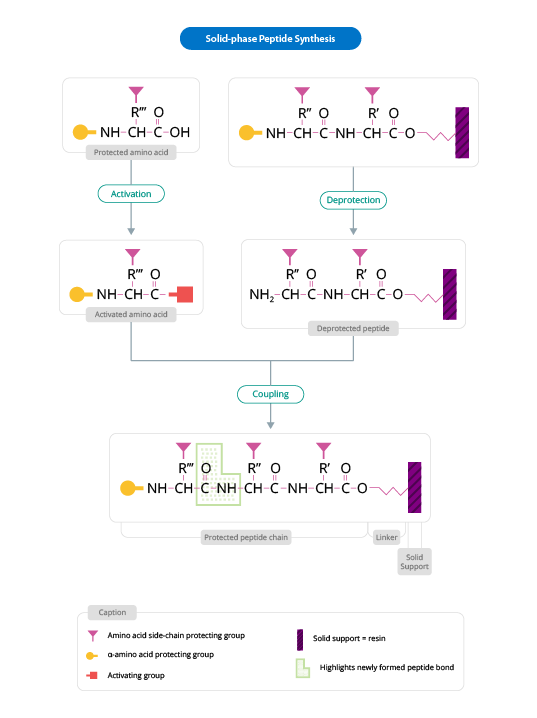
Figure 1: General Scheme for Solid Phase Peptide Synthesis
(Adapted from www.biotech.uiuc.edu)
The solid support is a synthetic polymer that becomes attached to groups of reagents with -OH. These groups are designed so that they can easily react with the carboxyl group of an N-α-amino acid, protected at its carboxyl terminus; it is thus covalently attached to the polymer. The protection of the amine group (X) may be removed and a second N-α-amino acid will become attached to the anchored amino acid. The steps described are repeated until the desired sequence is obtained. When the synthesis has finished, a reagent releases the carboxyl terminus from its attachment to the matrix, leaving the required peptide in solution.
The two main chemical processes used in peptide synthesis are Fmoc (Figure 2) and t-Boc (Figure 3).
Each method needs different protection of the amino acids linked to the chain and therefore different methods of cleavage/deprotection. Each method requires different resins. The main differences between the two systems are as follows:
| t-Boc | Fmoc | |
| Requires special equipment | Yes | No |
| Cost of reagents | Low | High |
| Solubility of peptides | Higher | Lower |
| Purity of hydrophobic peptides | High | May be lower |
| Problems with aggregation | Less frequently | More frequently |
| Synthesis time | ~ 20 min/aa | ~ 20-60 min/aa |
| Final deprotection | HF | TFA |
| Safety | Potentially dangerous | Relatively safe |
| Orthogonal | No | Yes |
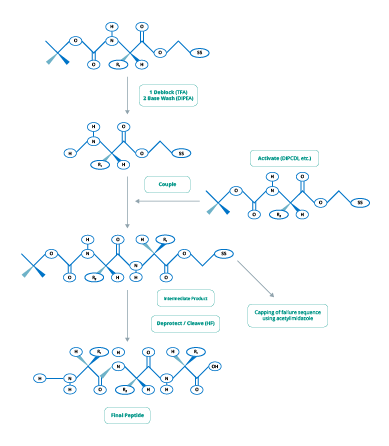
Figure 2. Fmoc-Chemistry for Peptide Synthesis
(Adapted from www.blog-biosyn.com)
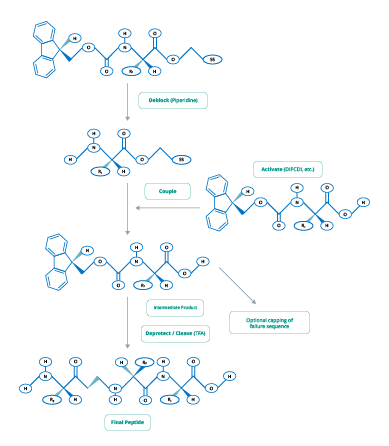
Figure 3. tBoc-Chemistry for Peptide Synthesis
(Adapted from www.blog-biosyn.com)
Peptide Design
Peptide synthesis can be simple, but a series of factors must be respected before commencing the synthesis. The sequence, composition of amino acids and length of peptides can influence the correct assembly of amino acids, and also later purification. These factors will affect the solubility of the final product. When designing peptides, the following points should be taken into account so that our customers receive the ideal product to suit their needs. At IMMUNOSTEP we offer customers our valuable experience in making their own designs a reality.
Composition of amino acids.
This affects not only ease ofassembly of amino acids, but also solubility, stability and the later purification process. Most peptides of interest, such as those used as antigens, proceed from N-term ends, C-term or from the interior of a sequence of native proteins. Thus these peptides are not always ideal for the customer\’s research purposes. Some can be insoluble or unstable, others can cause purification problems, whilst, in other cases, folding may not be similar to that of native proteins. Thanks to the experience we have acquired over the years, at IMMUNOSTEP we can locate those problematic amino acids and modify themby improving the qualities of synthesised peptides. Several different modifications can be made during productionaccording to customers\’ needs, for example covering N-term or C-term regions, as required, carrying out chemical modifications, inserting spacers, or replacing “problematic” amino acids.
Length of Sequence.
One of the most critical factors in synthesis and purification is excessive length. For instance, at IMMUNOSTEP we recommend never exceeding 10-15 amino acids for peptides used as antigens, since they will be ideal as they maintain the structure of the linear epitope in native proteins.
Hydrophobic Zones.
Hydrophobicity affects peptide solubility considerably; in this sense, our experience allows us to increase peptide solubility by adapting the frequency of the different amino acids depending on their hydrophilic and/or hydrophobic characteristics. High incidence peptides such as tryptophan, leucine, valine, methionine, phenylalanine, isoleucinemay not dissolve in aqueous solutions, and this allows us to maintain the ratio of these hydrophobic amino acids below 50% while securing the existence of at least one charged amino acid per five residues. At physiological pH, arginine, glutamine, aspartic acid and lysine are charged on their side chains. One simple change or the elimination of some polar residues at the N-term or C-term ends may influence solubility either positively or negatively.
Problematic amino acids:
cysteine, methionine and tryptophan can be prone to oxidation on their side chains, hence peptides which contain a percentage of these can make obtaining a high degree of purity difficult. Thanks to our experience in peptide synthesis, we are able to prevent such oxidation and guarantee high-purity peptides.
Secondary Structure.
During the process of peptide synthesis, the formation of β sheets may affect the solvation and ordered synthesis of the peptide, causing losses in its different zones. We can reduce the number of these deletions and the appearance of unwanted products in the synthesis by recommending the elimination of zones in which there are multiple or adjacent residues of valine, isoleucine, tyrosine, phenylalanine, tryptophan, leucine, glutamine or threonine. In most cases, conservative changes, as well as other measures applied during synthesis can improve chances of success with regard to purity and quality. Measures such as the insertion of glycine or proline, every three residues, or the replacement of glutamine for asparagine, or threonine for serine.
Peptide Modification
We often find that our customers require diverse modifications to the peptides which have already been developed. Such modifications are common in different experiments and require the experience of a group that is accustomed to dealing with them.
How can we help you?
IMMUNOSTEP \’s experience in the development of peptide synthesis has been the key to our customers\’ success in their research projects. This has enabled us to offer a wide range of products for peptide synthesis and modification. In our nearly 15 years of experience, we have designed and synthesized a multitude of peptides that have been used by our customers in virtually all of their areas of work, most especially in research.
| Nº | Service | Description |
| 1 | Synthesis of families of peptides for studies of transportation in membranes. | Design and synthesis of families of peptides that transport HIV in established lines. In vitro studies of cell infection. |
| 2 | Peptide synthesis for use in the development of antibodies (Polyclonal and Monoclonal). | Design, synthesis, modifications and conjugation to carrier proteins for the utilisation of sequences which may be employed as a target for the development of antibodies, both polyclonal in different species (rabbit, chicken, mouse…) and monoclonal (mouse). |
| 3 | Peptide synthesis for in vivo studies. | Design and synthesis of peptides for in vivo studies with animal models, murine, in the development of neurodegenerative diseases. |
| 4 | Design and synthesis of peptides for the development of diagnostic kits (IVD). | Development of peptides for the study of diseases of great social impact- Studies of Coeliac Disease- Immunogenic sequences in gluten. |





