TransFix® - Human Blood Stabilisation
TransFix®: A Stabilisation Solution that Prevents Cellular Degradation in Whole Blood for Flow Cytometric Testing
Without stabilisation, flow cytometric analysis of blood samples must be performed within 48 hours of venepuncture, as apoptosis and proteolytic degradation of cellular markers will detrimentally affect the immunophenotypic profile. Blood samples older than 48 hours are often no longer suitable for flow cytometric examination, exhibiting indistinguishable cell subsets and inaccurate absolute cell counts leading to erroneous clinical results. This may require a fresh sample to be taken, incurring extra costs and delayed analysis.
Features of TransFix
TransFix is a solution that stabilises leucocytes and leucocytic antigens for up to 14 days, preserving the absolute cell counts found in fresh blood and allowing differentiation of leucocyte sub-populations. This allows for an extended time frame for analytical testing.
Multiple product formats are available. The following products are CE-marked as in vitro Diagnostic Medical Devices (IVDs) in Europe for the purposes of blood cell stabilisation: TransFix/EDTA Vacuum Blood Collection Tubes, TransFix Sample Storage Tubes, and TransFix Bulk Reagent.
TransFix/EDTA Vacuum Blood Collection Tubes are also under FDA review for sale as IVDs in the USA.
Benefits of TransFix
• Prevents leucocytes and leucocytic marker degradation for up to 14 days:
– Greater flexibility in the laboratory.
– Ability to batch samples prior to analysis.
– Time for samples to be transported to/from remote sites.
– Reduction in the requirements of repeat sampling due to unusable samples.
– Accommodates for staff shortages or machine breakdown.
• Absolute cell counts of stabilised samples equivalent to fresh blood.
• Equivalent immunophenotypic profile to fresh blood with easily defined leucocyte sub-population (minimal adjustment of automatic gating strategies required).
• Prevents cellular degradation: Low levels of debris.
• Apoptopic pathways blocked preserving cellular morphology (1)
• Easy to use, just add TransFix and mix by inversion.
• Multiple product formats available.
Applications of TransFix
Clinical Use
The active components of TransFix stabilise leucocytes and leucocytic antigens for up to 14 days. The intended use of TransFix is the immediate stabilisation of blood samples, ready for flow cytometric testing, immune monitoring and immunohaematology screening purposes.
Immunophenotyping and diagnosis of haematological malignancies (e.g. leukaemia) by flow cytometry
Leukaemia is a malignant progressive disease where bone marrow and other blood-forming organs produce increased numbers of immature or abnormal leucocytes.
Each class of leucocytes express a unique combination of antigens on the cell surface (CD markers). A pathological cell may express more or less of a particular marker than a healthy cell, or additional CD markers.
It is important for clinicians to know what markers are expressed on pathological cells to get an accurate diagnosis. The time taken to perform ‘primary analysis’, (i.e. the initial screen) means that the sample is often not fresh enough to undergo detailed phenotyping at a later date. By stabilising samples at the point of collection, the immunophenotype at this time point is preserved.
See Case Study 1, 2, 3
Immune Monitoring of HIV/AIDS Patients
Monitoring CD4 and CD8 T-cell subsets of HIV-infected individuals is performed routinely by flow cytometry. As the disease progresses, the number of CD4+ cells reduce, destroyed by CD8+ T cells. Ordinarily, specimens are processed within 36 hours to obtain reliable CD4+ T-cell absolute counts.
By stabilising human whole blood, samples can be shipped at ambient temperature for up to 4 days. This is particularly important in countries where clinics are remote and the transport infrastructure is unreliable.
See Case Study 4, 5, 6
An independent study has also shown that TransFix is a highly effective anti-viral agent that results in a reduction in HIV replication as measured by HIV p24 antigen production (Case Study 7).
Benefits of Stabilising Whole Blood for Immunophenotyping
• Flexibility in Analysis
– Eliminates out of hours work- Ability to batch samples
– Reduces impact of unexpected machine breakdown and staff shortages.
• Ability to Ship Samples
– Samples can be shipped over long distances to reference laboratories without requiring cold chain transportation which provides significant cost savings
– Large batches of samples can be shipped together, reducing the demand to ship frequently to centralised laboratories.
• Reduction in False Negative Results (aberrant cell populations are detected)
– Fewer patient recalls due to unusable sample
– Earlier diagnosis and treatment
– Time saved to analyse more samples
• Enable Repeat Analysis without Patient Recall
– Further tests can be performed after the initial analysis if there was an abnormal result
– Eliminate the need for patient recall speeds up diagnosis.
Preparing a Blood Control
Preparing Blood Controls for Immunophenotyping – Towards Meeting the ISO15189:2012 Standard in the Clinical Flow Laboratory
The ISO standard entitled ‘ISO 15189: Medical laboratories – Particular requirements for quality and competence‘, refers to the ‘use of quality control materials that react to the examining system in a manner as close as possible to patient samples‘ (section 5.6.2.2), and that ‘the use of independent third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer ‘(section 5.6.2.2, Note 2).
It is common practice in immunophenotyping and immune monitoring laboratories to use a patient sample as a daily or even weekly control for flow cytometry. In addition, when a rare immunophenotype is detected there is an opportunity to preserve it for teaching or comparison purposes.
All samples left untreated are subject to apoptosis of cells and proteolytic degradation leading to immediate changes in the phenotypic profile. This poses a challenge, not only with analysing the sample in a short time scale but when preserving samples of interest for re-testing.
Simple addition of TransFix to a blood sample enables stabilisation of the immunophenotype for up to 14 days. Subject to validation at a clinical flow facility, this could provide a solution when such reference material is not available and would address the clauses referenced above.
Therefore, using TransFix, it is possible to stabilise blood samples (diseased and healthy) for use as a reference tool during flow cytometry analysis.
Protocol – Preparing a Blood Control
1. Collect blood by venepuncture into an EDTA vacuum tube according to CLSI document H3-A62.
2. Carefully remove the blood collection tube cap and determine the volume of anti-coagulated whole blood within the vacuum tube.
3. Pipette into the blood collection tube the appropriate volume of TransFix at the ratio of 0.2ml TransFix per 1ml of blood. For TransFix Sample Storage Tubes, add 1ml blood to the tube. Note: Blood samples should be treated with TransFix immediately after collection, but failing this, blood must be less than 6 hours old when it is treated with TransFix. Do not refrigerate the sample before treatment with TransFix.
4. Replace the cap on the blood collection tube, ensuring that there is no leakage and mix gently by inversion at least 10 times. Inadequate or delayed mixing may result in inaccurate test results. Do not vortex.
5. Once TransFix treated, the stabilised sample can be transferred to smaller test tubes to prevent repetitive cooling and warming thereby preserving the phenotypic profile of the sample.
6. Store/transport the TransFix treated blood for up to 14 days at 2 – 8°C or for up to 4 days at 18 – 25°C.
7. If refrigerated, incubate the treated blood sample at room temperature (18 – 25°C) for 15 minutes prior to use. Then mix the treated blood by rolling the tube between the hands 10 times and by inverting. Take care when opening blood collection tubes and briefly spin TransFix Sample Storage Tubes to reduce the build-up of blood in the caps.
8. Perform analysis by flow cytometry in accordance with the manufacturer’s instructions. A ‘stain, lyse no wash’ sample preparation method is recommended. Blood stabilised by TransFix should be analysed within 6 hours before being returned to 2 – 8°C storage for future use, if necessary.
Note a: Light scatter positions of cells stabilised by TransFix may differ slightly from those of untreated cells.
Note b: The dilution factor must be accounted for when calculating absolute cell counts. This can be done by multiplying the value given by the manufacturer to the absolute counting beads by 1.2 so that absolute cell counts are automatically corrected for TransFix treated blood samples.
Note c: Studies have shown that moderately high levels of haemolysis, icterus and lipemia do not affect the results. Grossly haemolysed samples should be rejected.
Clinical Research Organisations
Sample Storage and Transportation
TransFix provides a major benefit for Clinical Research Organisations, especially in the case of multicentre studies, where samples are routinely transported between collection sites and an analytical laboratory.
TransFix stabilised samples can be stored and transported at ambient temperature (18-25°C), removing the requirement for cold chain transportation, providing significant cost reduction (2).
Many clinical trials will collect samples from different sites. TransFix allows samples to be shipped to a single testing facility, reducing analyser variability.
Clinical trials often collect clinical samples over multiple time points. Batching large numbers of samples prior to transportation can significantly reduce trial costs.
When storing or shipping clinical samples it is essential that sample integrity is preserved.
Benefits of TransFix for Clinical Research Organisations:
• Improve data quality over transit, where immediate analysis is not possible.
• Standardisation of samples across multiple sites (all samples preserved at the same time point, all analysis can be performed at the same site).
• Batch samples for high-throughput analysis.
• Financial savings: Expensive transportation (cold chain storage) is not required.
• Single tube pack sizes available.
Cytomark provide a TransFix Phlebotomy Pack, which provides an individually wrapped TransFix/EDTA Vacuum Blood Collection Tube, with a safety holder with integrated and orientated needle. These are available in multiple needle sizes and draw volumes, and are designed for ease of use in collecting TransFix-stabilised human blood by CROs during clinical trials.
TransFix Performance Data
Transfix has been shown to stabilise human whole blood for up to 14 days prior to immunophenotyping. We have increased our claim from 10 days to 14 days following the results obtained from a 2-part evaluation in a clinical setting at 3 independent external sites.
Product Performance
Each clinical site recruited a number of healthy, leukemic, and HIV positive subjects.
TransFix stabilised peripheral blood was analysed for a full lymphocyte count (CD3, CD4, CD8, CD19, CD16/56 and CD45 panel) in duplicate, on a BD FACS Canto II flow cytometer (FCM). Samples were analysed at day 0 (<6 hours from collection), day 11, and day 15, and compared to the “gold standard” (which is blood collected in K3EDTA Vacutainers and tested within 6 hours).
Cell absolute counts within TransFix stabilised blood samples were found to be statistically equivalent to fresh blood. Additionally, subpopulations of lymphocytes were easily identified, including clearly segregated CD3 +/-ve populations and CD4+ and CD8+ T cell populations, demonstrating that TransFix can preserve human whole blood for up to 14 days at 2-8°C.
Flow cytometry dot plots demonstrating equivalence between fresh blood and TransFix stabilised blood
Figure 1: Gold Standard (fresh blood in K3EDTA Vacutainer)
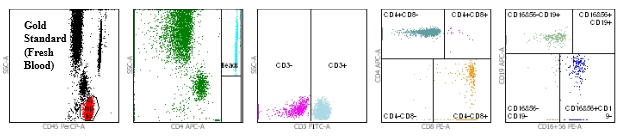
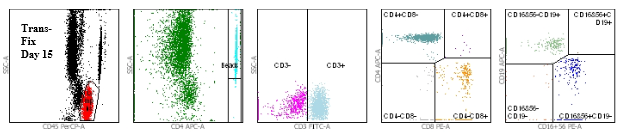
Figure 2: TransFix stabilised blood (Day 15)
Figures 1 and 2 show the flow cytometry dot plots for a HIV positive subject. Figure 1 shows the flow cytometry gating for the “gold standard” control (Vacutainer, Day 0) specimen for cell markers CD3, CD4, CD8, CD19, CD16+56, CD45. Figure 2 shows the flow cytometry gating for the TransFix stabilised blood on Day 15 for the same cell markers. The plots show that the immnophenotype is maintaining when compared to the fresh blood sample.
Competitor Comparison
TransFix stabilised blood has been compared to blood stabilised by a competitor reagent and the gold standard (fresh blood in a 4ml BD Vacutainer). The resulting dot plots are shown in figures 3, 4, and 5. TransFix shows equivalent leucocytic profiles to the fresh blood control at Day 15, with low levels of cellular debris, good separation of CD3+/-ve populations, and similar mean fluorescence intensities to fresh blood allowing for clear segregated of leucocyte sub-populations in TransFix treated samples.
Flow cytometry dot plots for fresh blood, competitor reagent stabilised blood, and TransFix stabilised blood
Figure 3: Gold Standard (fresh blood in BD K3EDTA Vacutainer)
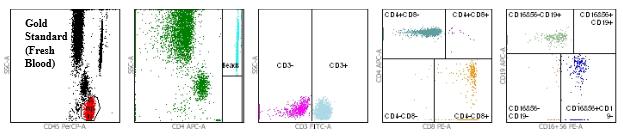
Figure 4: Blood stabilised with competitor product (Day 15)

Figure 5: TransFix stabilised blood (Day 15)

Figures 3-5 show the flow cytometry dot plots for a HIV subject. Figure 3 shows the flow cytometry gating for the gold standard control (4mL BD Vacutainer, Day 0) tube for cell markers CD3, CD4, CD8, CD19, CD16+56, CD45. Figure 4 shows the flow cytometry gating for the stabilised blood using a competitor product, Day 15. Figure 5 shows the flow cytometry gating for TransFix stabilised blood, Day 15.
Interference
The stabilising properties of TransFix are not affected by high levels of bilirubin, triglycerides, or haemoglobin. Interference substances studies show a good agreement between TransFix stabilised blood sample cell counts and equivalent samples spiked with interfering substances.
Reproducibility
TransFix is manufactured and quality controlled under strict guidelines according to ISO 13485. Precision analysis showed good reproducibility between the three lots, over 54 patient samples.
Case Studies
The following case studies present examples of the uses of TransFix. Publications that cite the use of TransFix to stabilise whole human blood can be viewed in our bibliography.
Case Study 1: TransFix: A clinical sample stabilising fluid for use in clinical haematology and immunology
TransFix was developed by UK NEQAS as a stabilising solution which retains sample integrity for up to 10 days. The study examined 40 patients specimens treated with TransFix and found no loss of antigenicity over a 10 day period. A full haematological profile (including differential) was obtainable at day 7.
Reference: Barnett et al (1999). TransFix: A clinical sample stabilising fluid for use in clinical haematology and immunology. Cytometry 38: 88
Case Study 2: Evaluation of leukocyte stabilisation in TransFix treated blood samples by flow cytometry
The study evaluated the effects of TransFix on leucocyte scatter characteristics, immunophenotyping, membrane permeability, absolute cell counting and morphology. The results showed that this short term stabilisation method is particularly suitable for the analysis of human lymphocytes. Good results were obtained with respect to antigen definition and absolute cell counting procedures.
Reference: Canonico et al (2004). Evaluation of leukocyte stabilisation in TransFix treated blood samples by flow cytometry and transmission electron microscopy. Journal of Immunological Methods 295: 67-78
Case Study 3: Flow cytometric profiles, biomolecular and morphological aspects of transfixed leukocytes and red cells
Cytometric performance is suboptimal in aged unfixed specimens because of apoptosis that affects light scatter properties. TransFix is able to abolish any apoptotic features and acts as an optimal blood preservative for appropriate preanalytical stabilisation.
Reference: Canonico et al (2010). Flow cytometric profiles, biomolecular and morphological aspects of transfixed leukocytes and red cells. Cytometry B, Clinical Cytometry 78(4): 267-78
Case Study 4: CD4 Count in a Community-Based Study From Guinea-Bissau, West Africa
This study showed that TransFix added to fresh blood demonstrated excellent results for lymphocyte subset measuring when transported to a centralised laboratory.
Reference: Henig et al (2011). CD4 Intragenic SNPs Associate With HIV-2 Plasma Viral Load and CD4 Count in a Community-Based Study From Guinea-Bissau, West Africa. Journal of Acquired Immune Deficiency Syndromes 56: 1-8
Case Study 5: Advances in CD4 cell enumeration
The availability of antiretroviral medications increases the need for CD4 cell assays to monitor disease progression and the efficacy of therapy in resource-poor countries. Simplified flow cytometry is needed in centralised clinics, but there is a critical need for even less expensive easier methodology that can be used in smaller laboratories and in rural villages. TransFix can be used to stabilise samples for transportation to central laboratories.
Reference: Baum et al (2007). Advances in CD4 cell enumeration in resource-poor countries. Current Opinion in HIV and AIDS 2: 157-245
Case Study 6: Performance of aged, TransFix treated blood in CD4 and CD8 Assays
The study reported the compatibility of 15 day old TransFix treated blood with the Guava EasyCD4 and EasyCD8 assays. The staining pattern for TransFixed samples over 15 days was almost identical to the staining pattern at day 0.
Reference: Mergai et al (2005). Performance of aged, TransFix treated blood in the Guava EasyCD4 and EasyCD8 Assays. 12th conference on retroviruses and opportunistic infection.
Case Study 7: TransFix has been shown to inhibit HIV replication
To study the effect of TransFix on reduction of HIV replication, CEM 13D cells (15x106cells) were infected with various dilutions of subtype B HIV-1. Cultures were then treated with diluted TransFix and incubated for 7 days. The results obtained within 24 hours of treatment with TransFix showed a 1-log reduction in HIV replication as measured by HIV p24 Ag production. The results indicate that TransFix has the ability to cause significant reduction of HIV replication.
Reference: Kim et al (2002). Evaluation of TransFix, a commercial whole blood stabilizing reagent. This product reduces HIV replication. Clinical Cytometry 50: 281
*Cytomark has not independently verified these findings. Cytomark does not take liability if TransFix is used in applications other than those validated by Cytomark.
Product Information
Several formats of TransFix are available for human blood stabilisation:
TransFix/EDTA Vacuum Blood Collection Tubes (CE/IVD)
TransFix/EDTA Vacuum Blood Collection Tubes are plastic, direct draw collection tubes used to stabilise venous blood at the point of collection and preserve whole blood specimens for immunophenotyping by flow cytometry.
Tubes are available in 3ml or 9ml sizes. Each tube is prefilled with sufficient TransFix containing K3EDTA for the stabilisation of 3ml or 9ml of blood, respectively. TransFix/EDTA Vacuum Blood Collection Tubes are available in pack sizes of 2 or 50 tubes. *TransFix/EDTA Vacuum Blood Collection Tubes are designed, manufactured and CE marked for IVD use by Vacutest KIMA s.r.l, Italy and contain Cytomark CE/IVD marked TransFix.
TransFix Sample Storage Tubes (CE/IVD)
TransFix Sample Storage Tubes consist of 1.2ml tubes containing 0.2ml of TransFix. These tubes do not contain an anti-coagulant.
| Description | Code |
| TransFix Sample Storage Tubes (2 x 1.2ml tubes) | TF-01-2 |
| CE/IVD (2 x 1.2ml tubes) | |
| TransFix Sample Storage Tubes (2 x 1.2ml tubes) | TF-01-2-RUO |
| RUO (2 tubes) | |
| TransFix Sample Storage Tubes (10 x 1.2ml tubes) | TF-01-10 |
| CE/IVD (10 x 1.2ml tubes) | |
| TransFix Sample Storage Tubes (10 x 1.2ml tubes) | TF-01-10-RUO |
| RUO (10 tubes) | |
| TransFix Sample Storage Tubes (25 x 1.2ml tubes) | TF-01-25 |
| CE/IVD (25 x 5ml tubes) | |
| TransFix Sample Storage Tubes (25 x 1.2ml tubes) | TF-01-25-RUO |
| RUO (25 tubes) | |
| TransFix Sample Storage Tubes (50 x 1.2ml tubes) | TF-01-50 |
| CE/IVD (50 x 1.2ml tubes) | |
| TransFix Sample Storage Tubes (50 x 1.2ml tubes) | TF-01-50-RUO |
| RUO (50 tubes) |
TransFix (CE/IVD)
TransFix is provided in 1 and 20ml aliquot sizes and in pack sizes of 10 and 50 1ml tubes. This gives the end user most flexibility with regard to application of TransFix to research samples.
| Description | Code |
| 1ml TransFix | TFB-01-1 |
| CE/IVD (1ml) | |
| 1ml TransFix | TFB-01-1-RUO |
| RUO (1ml) | |
| 10 x 1ml TransFix | TFB-01-10 |
| CE/IVD (10 tubes) | |
| 10 x 1ml TransFix | TFB-01-10-RUO |
| RUO (10 tubes) | |
| 50 x 1ml TransFix | TFB-01-50 |
| CE/IVD (50 tubes) | |
| 50 x 1ml TransFix | TFB-01-50-RUO |
| RUO (50 tubes) | |
| 20ml TransFix | TFB-20-1 |
| CE/IVD (20ml) | |
| 20ml TransFix | TFB-20-1-RUO |
| RUO (20ml) |





